Optimization and Validation of LC/MS Methods
Formats: self-paced
Length: Self-paced: Nine recorded sessions
Fee: $1,500 USD
![]()
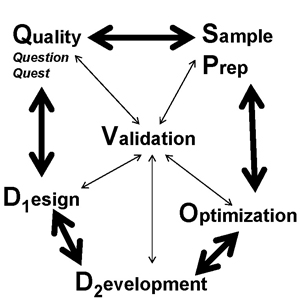 We are pleased to announce a new and unique training course, Optimization and Validation of LC/MS Methods. This new course will provide attendees not only with the knowledge necessary to develop useful LC/MS methods but also the procedures needed to ensure the quality, robustness, transferability and regulatory compliance of those methods.
We are pleased to announce a new and unique training course, Optimization and Validation of LC/MS Methods. This new course will provide attendees not only with the knowledge necessary to develop useful LC/MS methods but also the procedures needed to ensure the quality, robustness, transferability and regulatory compliance of those methods.
LC/MS in the 1990s was still a tool primarily for researchers which required specialized knowledge to operate successfully and to interpret the data. Since the beginning of this century, LC/MS has rapidly evolved with new interfaces, new mass analyzers, and new software. These developments have made LC/MS accessible to all analytical chemists and have allowed what was once a research tool to move into product development and quality assurance laboratories. It is easy to see that LC/MS will become an important tool in quality control and in manufacturing in the near future.
With these new capabilities, come new responsibilities. LC/MS users must not simply develop “one-off” methods, but must ensure that the technology can be used in regulated environments, complying with GLP and GMP standards and satisfying regulatory agency requirements. This course will focus on the technology of LC/MS, and take a detailed look at method development all the while discussing these topics in the context of quality systems and method validation.
The two instructor format brings different expert perspectives on the problems of method development and validation. Extensive audio-visuals support the lecture content and attendees receive a printed manual including all graphics presented in the course. Attendees are encouraged to interact with the instructors throughout the class and concepts are solidified by a final homework assignment which is discussed in the last session.
The key topics to be presented include:
- How ions are formed in the LC/MS interface and what happens to them in the mass spectrometer
- Key points to understanding and making the best use of mass spectra data
- The key steps and important considerations when developing and optimizing an LC/MS method
- A pathway for developing LC/MS methods with a Quality by Design (QbD) approach; understanding the goals and scope of the method
- Risk Assessment strategy; Relevance to methods development & systems
- The relevance of System Suitability vs. risk assessment for LC/MS methods
- Understanding Qualification and Validation of LC/MS systems (IQ, OQ, PQ)
- Preparation of Analytical Data packages for submission to FDA and other Regulatory Agencies
Benefits of attending this class are:
- Understanding the operation of and considerations related to LC/MS systems and the use of this understanding to produce optimized mass spectral data and methods
- Finding out what is required of the laboratory scientist and the management team to meet regulatory expectations and to ensure the production of top quality LC/MS data
- Learning from two instructors with over 75 years of combined experience; looking at LC/MS method development from the science as well as the quality perspective
Who Should Attend
- Analytical chemists and laboratory managers in regulated industries such as pharmaceuticals, diagnostics, biotechnology, cosmetics and food
- Those in government laboratories doing environmental and forensic analyses wich much withstand legal scrutiny
- Any chemist developing LC/MS methods in any industry, agency or school who is concerned with the validity of their methods and the quality of their results
Therefore, this course is for everyone who is presently using LC/MS, may use LC/MS in the future, or who must understand and evaluate data from LC/MS analyses.
Agenda:
Quality Concepts (related to Methods Development, Optimization and Validation)
- Quality: Laboratory QA/QC and Basics of Quality Systems
- Perspectives of Analytical Methods and Critical Quality Attributes (CQA)
- Relevance of Data and Data Integrity
- Measurement, Measurement Uncertainty and Error
- Understanding the Relevance of Validation for LC/MS Methods
Mass spectrometry
- What do mass spectrometers do?
- What does MS data look like and what can it tell us?
- What are the differences and similarities between various types of MS?
- Transmission Quadrupole
- 3D Quadrupole Ion Trap
- 2D Quadrupole Ion Trap (Linear Trap)
- Time-of-Flight
- Orbitrap
- Magnetic Ion Trap (FT-ICR)
LC/MS Interfaces
- What are the appropriate analyte types for each variety of LC/MS interface?
- How are ions formed inside an LC/MS interface?
- Electrospray (ESI)
- Atmospheric Pressure Chemical Ionization (APCI)
- Atmospheric Pressure Photoionization (APPI)
LC/MS Systems
- System configurations appropriate for particular analytical goals
- MS/MS
- Calibration and tuning of LC/MS systems
Development, Optimization and Validation Perspectives
- Rationale of Methods Development and Validation
- Guidelines for ab initio Methods Development, Optimization and Validation
- Generic Definitions for Validation Parameters
- International Conference on Harmonization (ICH) and USP Guidelines
- Regulatory Requirements
- Hurdles, Difficulties and Strategies for Methods Transfer
- What Comprises the Tech Transfer Packages?
Development and Optimization of LC/MS Methods
- Stepwise procedure for LC/MS method development
- Detailed summary of specific considerations for method optimization
- Flow rate
- HPLC column choice
- Mobile phase
- pH
- Temperature
- Voltages
- MS/MS
- Quantitative analysis
- LC/MS Methods Validation
- System Suitability Testing (SST)
- Developing Appropriate SST Requirements
- SST as Part of Methods Transfer Protocol
Homework Exercise: Summary of the Course Contents with "Real-World" Examples
Please note that every session is recorded and those recordings are made available to attendees within 24 hours of the completion of that session. Attendees retain access to the recordings for six months after completion of the class.
Instructors: Fred Klink and Shib Mookherjea
